Moderna developing booster shot for new coronavirus variants, increases vaccine production target | Reuters
FDA vaccine advisers 'disappointed' and 'angry' that early data about new Covid-19 booster shot wasn't presented for review last year | CNN

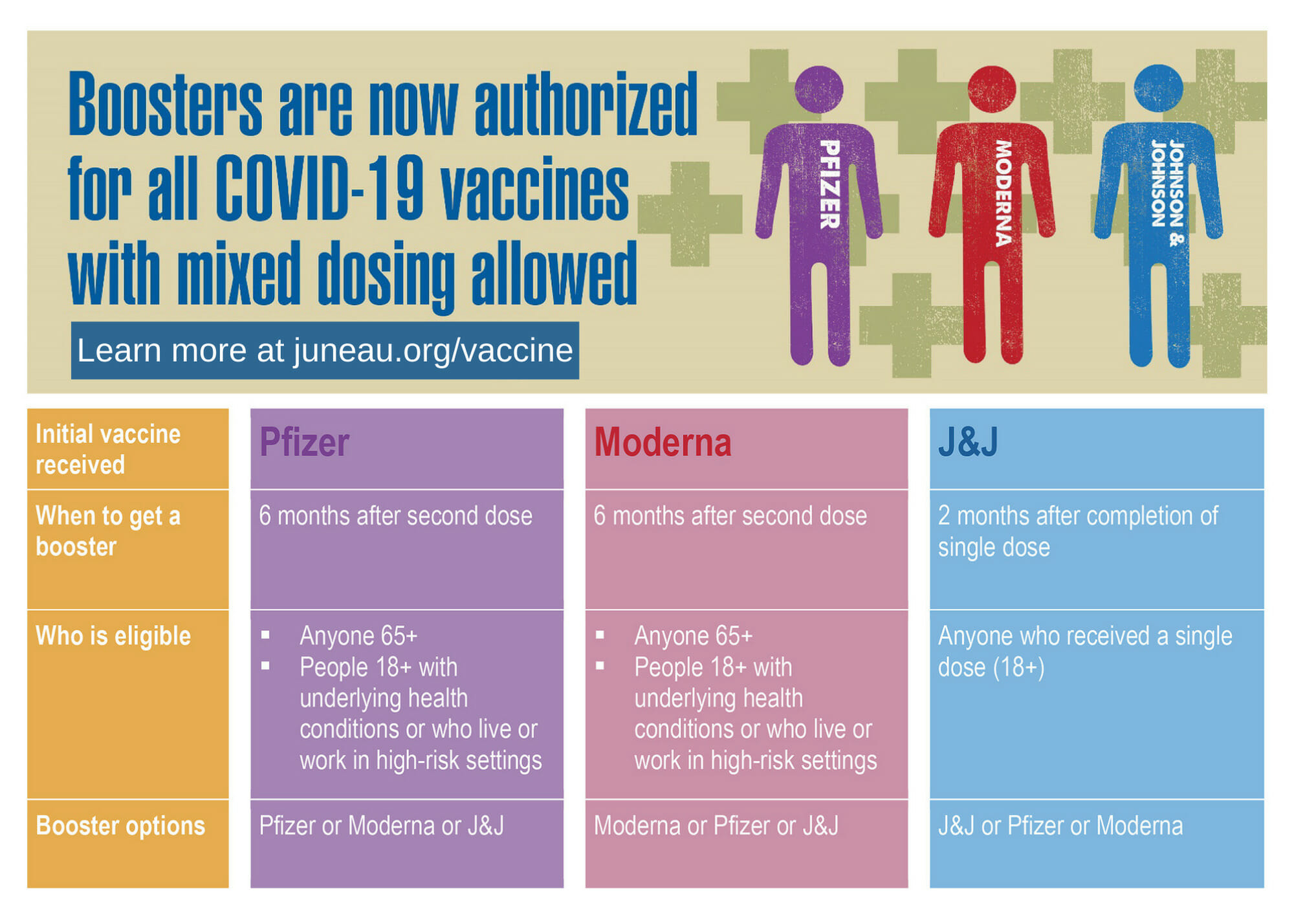
FDA authorizes another booster dose of the Pfizer or Moderna COVID-19 vaccine for people age 50 and up - The Boston Globe